Recently, an innovative design of anchoring nanoscale Pd on three dimensional carbon hybrid as highly active and stable catalyst for electro-oxidation of formic acid was completed by Yang Liang and his collaborators from School of Energy and Power Engineering. This groundbreaking research was published as a full article in Applied Catalysis B: Environmental, a top-level journal of energy and environment filed (Applied Catalysis B: Environmental 263(2020) 118304).
The electrochemical system such as fuel cell with the advantages of low pollutant emission and high specific energy density offer promising substitutes of fossil fuels to address the energy shortage and the environmental issues. However, the grand challenge leading to extensive use of electrocatalysts is the degradation of active species which results in poor durability and long-term performances of fuel cells. From most reported research, despite the fact that the use of carbon-based supports to stabilize and modify active metal species and thus realize superior electrocatalytic performance for formic acid oxidation (FAO) has been extensively explored, the corresponding stabilization/degradation mechanisms of active metal are still not fully understood, as the origin of active metal particle stabilization by supports and the immobilization-induced changes of these particles have not been fully clarified.
In their work, a chemically controlled process for synthesizing size-controlled Pd anchoring on the flower-like novel 3D carbon hybrid of boron-doped graphene with carbon nitride (BG-CN) as FA oxidation (FAO) electrocatalyst is presented, revealing that the tuned surface electronic and chemical properties of the above support enhance the activity, CO poisoning tolerance, and stability of Pd NPs compared to those of commercial Pd/C and reported Pd based catalysts. The origin of this behavior and catalyst intrinsic degradation/stabilization mechanisms are probed by identical-location transmission electron microscopy (IL-TEM) which enables the atomic-scale visual tracking of the anchoring effect at the identical location. It reveals that the excellent stabilization effect of carbon hybrid can reduce the particle detachment and agglomeration comparing with commercial Pd/C. Theoretical DFT calculations further suggest that carbon hybrid with tuned surface electronic properties provides anchor sites to trap the Pd atoms and facilitates the formation of Pd clusters at trapping (nucleation) sites.
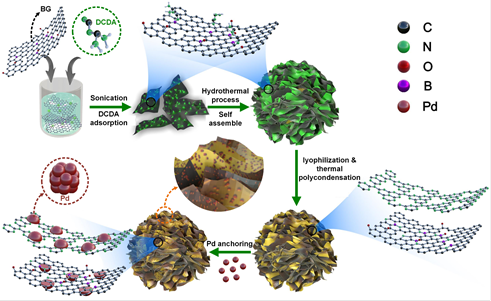
They believe that this work sheds light on the underexplored anchoring effect and stabilization mechanism of complex hybrid supports and provides new insights for the design and synthesis of hybrid catalysts with unique textural features and surface chemical properties for applications in energy conversion.
Source: Yang Liang, Wang Xin, Liu Daoping, Cui Guomin, Dou Binlin, Wang Juan. Efficient anchoring of nanoscale Pd on three-dimensional carbon hybrid as highly active and stable catalyst for electro-oxidation of formic acid. Applied Catalysis B: Environmental, 2020, 263: 118304.
https://www.sciencedirect.com/science/article/pii/S0926337319310501


