
Recently, Dr. Zhang Jie from School of Energy and Power Engineering, as the first writer and correspondent, published a research paper entitled “The evolution of catalytically active calcium catalyst during steam gasification of lignite char” in top journal Carbon on engineering technology together with Tang Jia, an engineer from Ningde Amperex Technology Limited, Liu Lijuan, a lecturer from School of Physics and Microelectronics, and Prof. Wang Jie from Department of Chemical Engineering for Energy of East China University of Science and Technology. The research proved the associative form and evolution of catalytically active calcium in producing hydrogen via gasification of lignite char.
Hydrogen is regarded as an ideal sustainable energy carrier with the feature of zero end-use emissions. Thus, various potential methods of hydrogen production were explored by using fossil fuel, biomass, photonic energy and so on. Although much recent work has been focused on hydrogen production via renewable energy and replenished resources (like water), it is imperative for countries where non-renewable energy contributed to most of their domestic energy demands to concentrate on the clean and efficient utilization of fossil fuels. Due to the well-developed process technology, coal gasification can be rapidly used in large-scale hydrogen production. Given the advantages of low operating temperature, low cost, high efficiency of energy conversion, and selective reaction pathways, the catalytic gasification of coal is one of the promising ways to produce hydrogen.
Dr. Zhang carried out studies on lignite char. The gasification of three different lignites and their pretreated samples showed that either the intrinsic calcium in lignite or extrinsic calcium added by ion exchange had high catalytic activity. It was proven that most of the calcium in the lignite was originally associated, which was mainly bound to carboxyl groups and highly dispersed at an atomic level. The changes of gasification rate, gas compositions, and oxygen chemisorption capacity with carbon conversion hinted that the catalytic mechanism of calcium was different from that of potassium. X-ray absorption near edge structure (XANES) analysis revealed that carboxyl-bound calcium was the active form of the catalyst, and the deactivation of the calcium catalyst in the gasification process was due to the transformation of carboxyl-bound calcium to other chemical forms. The organically bound calcium also led to the porous char structures at the early gasification stage, which improved the catalytic activity. The research result is of practical indicative meanings and theoretical values in subsequent research in hydrogen production by using lignite char.
The research is granted by Natural Science Cultivation Foundation and Startup Found at the University of Shanghai for Science and Technology (Grant No. ZR20PY05 and 1D-19-301-005), as well as is supported by research platform and research team of School of Energy and Power Engineering.
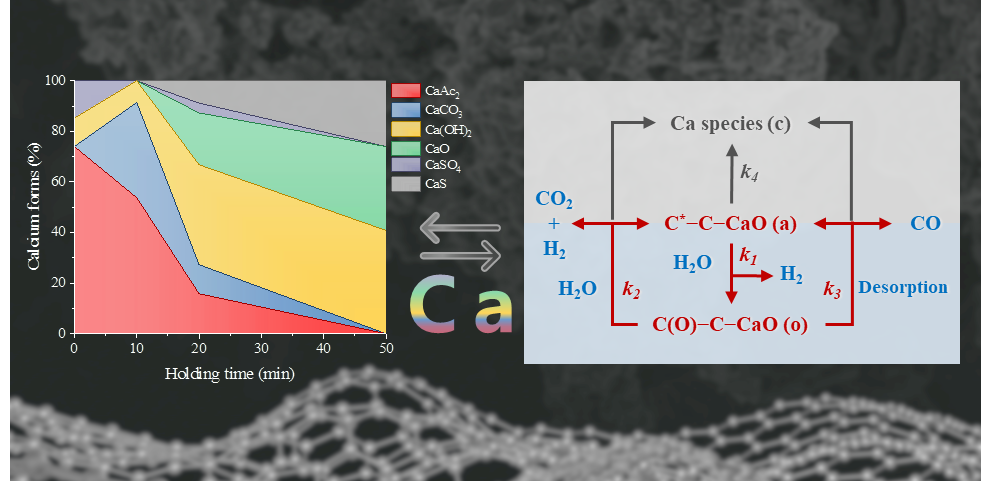
The evolution of calcium species via XANES and the simplified active site/intermediate mechanism of calcium-catalyzed char gasification.
Source from School of Energy and Power Engineering


